Meeting work and community engagement requirements should take into consideration areas of high unemployment or caregiving for young children or elderly family members. States will therefore be required to describe strategies to assist eligible individuals in meeting work and community engagement requirements and to link individuals to additional resources for job training, provided they do not use federal Medicaid funding to finance these services.
Healthcare.gov Special Enrollment Verification Phase II: Marriage, Adoption, Medicaid
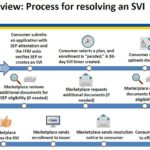
After a consumer has submitted an application under the Special Enrollment Period with a qualifying event, and then selects a health plan, the consumer will have 30 days to verify the qualifying event. The consumer can upload or mail the documentation verifying the qualifying event. The Marketplace will review the documentation and request additional information if necessary.
California Hospital Quality Ratings for 2016
Today, we [Centers for Medicare and Medicaid] are updating the star ratings on the Hospital Compare website to help millions of patients and their families learn about the quality of hospitals, compare facilities in their area side-by-side, and ask important questions about care quality when visiting a hospital or other health care provider. Today’s update comes after substantive discussions with hospitals and other stakeholders to review the Overall Hospital Quality Star Rating’s methodology.
2016 Medicare – Medicaid Dual Eligible Income Levels
Centers for Medicare & Medicaid Services (CMS) released an Informational Bulletin updating the poverty guidelines that are applied to eligibility criteria for programs such as Medicaid and the Children’s Health Insurance Program (CHIP). Included with this informational bulletin is the 2016 Dual Eligible Standards chart that displays the new standards for the Medicare Savings Program categories.
Feds urge drug makers and states to make Hepatitis C drugs available
Recognizing that we need both access and affordability, today we issued a notice to all 50 state Medicaid directors and sent letters to the CEOs of several drug manufacturers about providing access to therapy for Hepatitis C patients. Our notice to state Medicaid directors reminds states of their obligation to provide access to these promising therapies (consistent with section 1927 of the Social Security Act) based on the medical evidence, and that they have tools available to manage their costs. Our letter to manufacturers asks them to provide us with information on pricing arrangements and asks them for ideas to support the provision of these lifesaving medications to Medicaid programs at sustainable prices.